The oximeter requires 510(K)
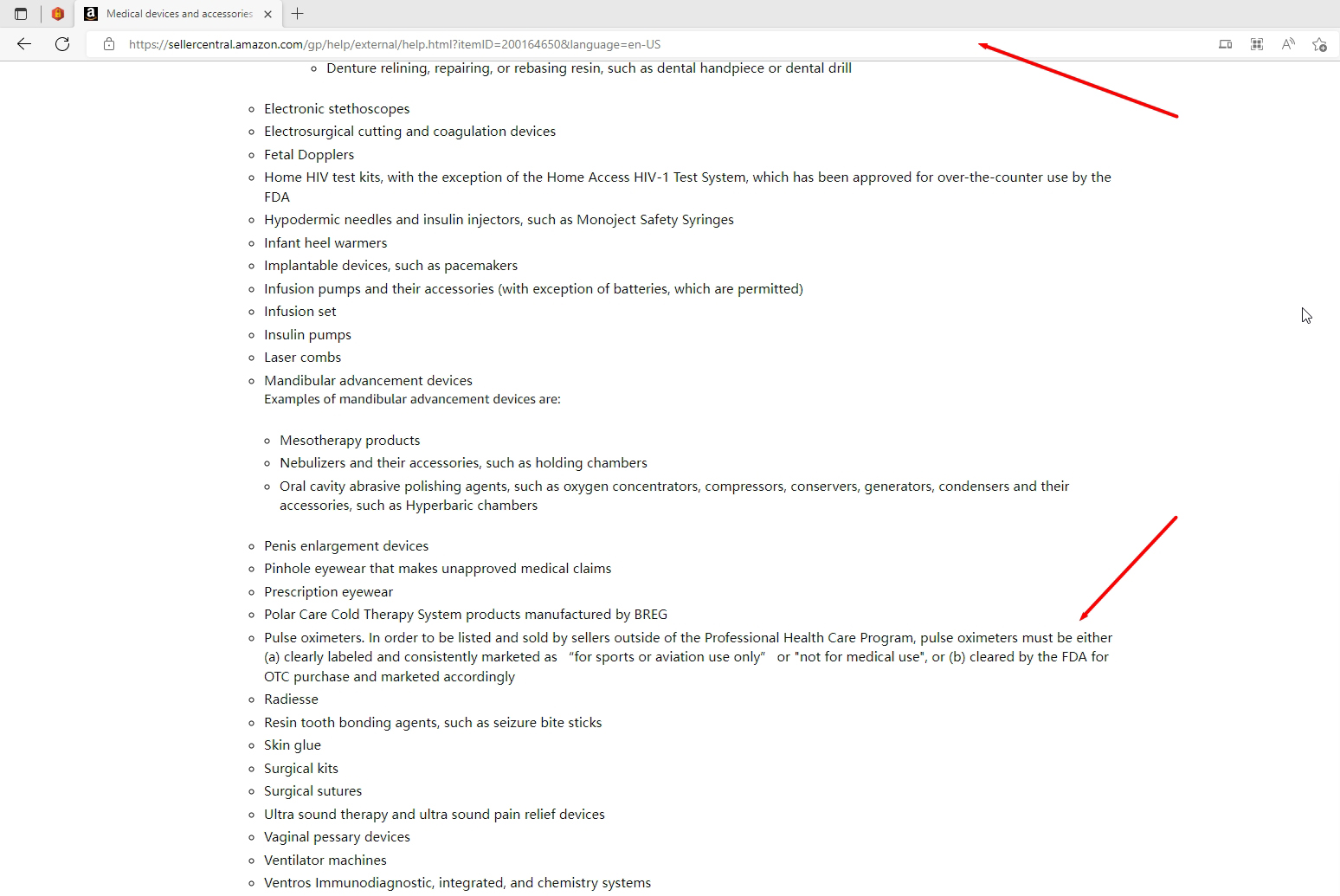
I sell oximeter in Amazon, this product does not require a 510 (k), Help page on medical devices statement:Pulse oximeters. In order to be listed and sold by sellers outside of the Professional Health Care Program, pulse oximeters must be either (a) clearly labeled and consistently marketed as "for sports or aviation use only" or "not for medical use", or (b) cleared by the FDA for OTC purchase and marketed accordingly
Help page on medical devices URL :https://sellercentral.amazon.com/gp/help/external/help.html? itemID=200164650&language=en-US
Product packaging and user manual declare "this device for sports or aviation use only, not for medical use.
However, the Amazon audit team repeatedly asked for 510(K). I feel helpless selling in Amazon Mall.
The oximeter requires 510(K)
I sell oximeter in Amazon, this product does not require a 510 (k), Help page on medical devices statement:Pulse oximeters. In order to be listed and sold by sellers outside of the Professional Health Care Program, pulse oximeters must be either (a) clearly labeled and consistently marketed as "for sports or aviation use only" or "not for medical use", or (b) cleared by the FDA for OTC purchase and marketed accordingly
Help page on medical devices URL :https://sellercentral.amazon.com/gp/help/external/help.html? itemID=200164650&language=en-US
Product packaging and user manual declare "this device for sports or aviation use only, not for medical use.
However, the Amazon audit team repeatedly asked for 510(K). I feel helpless selling in Amazon Mall.

0 replies
Glenn_Amazon
Greetings @Seller_mnIWWoFMmxdKn,
Thank you for reaching out with your concern. In some instances Amazon may request documentation if the detail product information or product packaging is not sufficient. If you provide your most recent Seller Support case on this issue I can review to see what further actions are available to contest the Medical devices and accessories restrictions on your product. Thank you.
-Glenn